Lisa
-


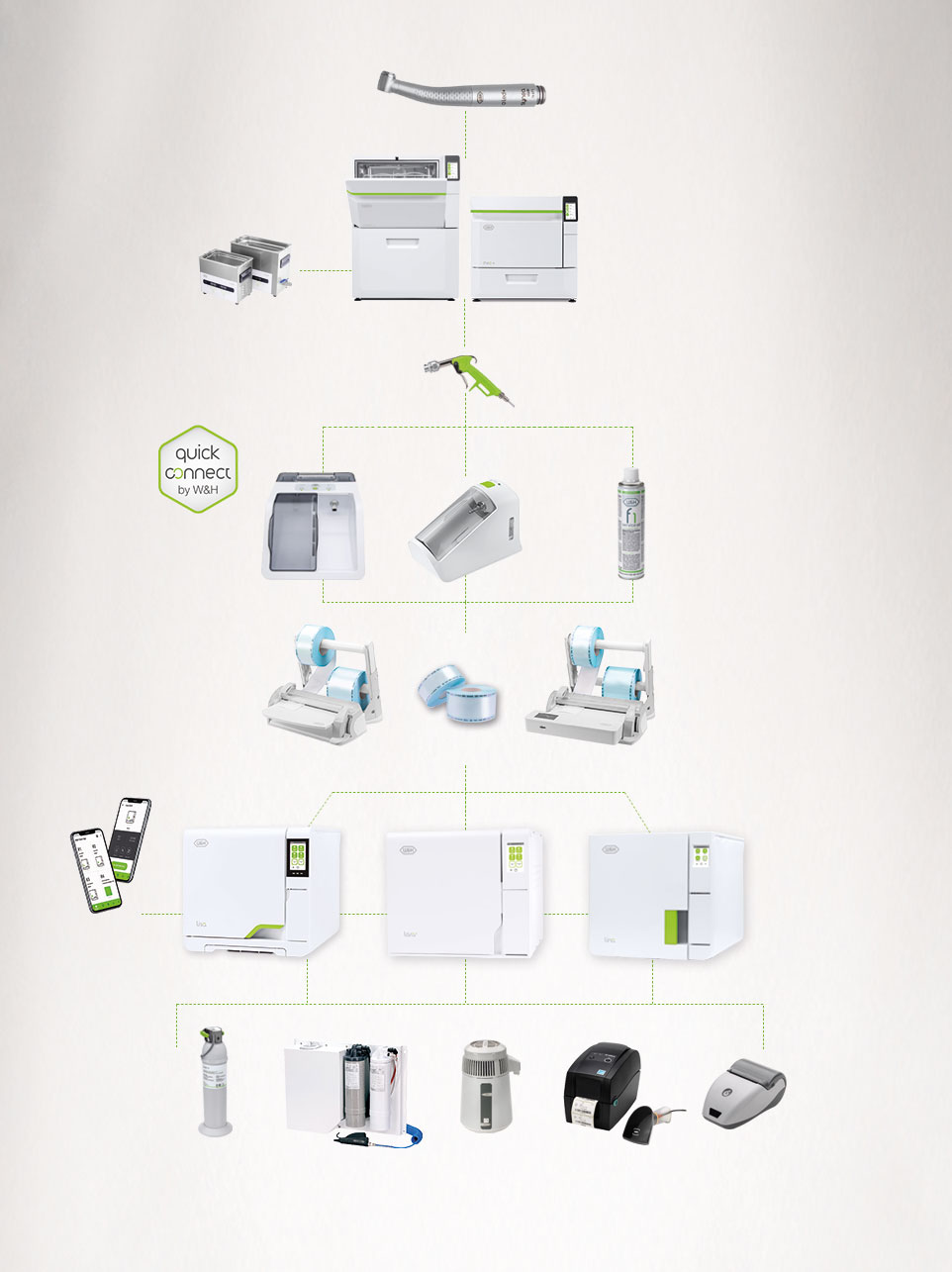
Reliable and safe sterilization with Lyla
In the reprocessing workflow, the sterilization of your dental instruments is deeply important.
That is why we aim for best standards.Lyla reduces the risk
of infection through its- Smart upgrades
- Super fast cycles
- Secure traceability
- Simple operation
-
New environmentally friendly packaging
The new environmentally friendly packaging is completely reusable and recyclable. Plastic components were reduced to a minimum. For 99% of its weight, the new package is made of cardboard and other fully biodegradable substances.

 The "B cycle”
The "B cycle”
feature upgrades Lyla to B type sterilizer according to
EN 13060.Upgradeable from S to B

The “B cycle” feature upgrades Lyla simply
and quick to a B type sterilizer according to
EN 13060.
 Remote data storage
Remote data storage
offers the possibility to save cycles directly on PC.Remote data Storage
The “Remote data storage” feature
is the ideal addition for the digital
cycle documentation.
 The “Traceability”
The “Traceability”
feature enables user identification and load release confirmation.Traceability

Identification is secured by a PIN
directly on the Lyla sterilizer and
the W&H Steri App.Six steps to your customized sterilizer:
- Purchase the Activation Code of your choice from your local approved dental supplier.
- After receiving the related login code, go to activation.wh.com
- Specify this code along with the serial number and model of your sterilizer.
- Your individual, secure Activation Code is generated.
- Enter this code in the menu of your sterilizer.
- The feature is available.

Compatibility:
The application is compatible with all sterilizers currently available.
Available languages: EN, IT, FR, DE, ES, ZH-CN, JP
Main features:
Remote control of your sterilizer: the user can remotely command up to 4 sterilizers.
Advanced traceability: EliTrace feature is now fully supported and the App permits to create instrument database directly from your smart device. In addition to this function, the management and tracking of dental instruments is integrated.
Reports creation: the App allows making intervention reports in order to collect data regarding specific treatments.
User management: creation and handling of sterilizer users directly from your smart device.
Backup functionality: all the cycle reports are automatically saved to assure more protection of data.
Lyla sterilizer
supplied with reversible rack, 3 aluminium trays, drain tube, tray holder, 8 GB USB drive, door opening tool, factory test,
50 HzLyla 17
REF 19951150Chamber size 17 litre
Lyla 22 (220 V)
REF 19952150Chamber size 22 litre
Lyla 22 (110 V)
REF 19952350Chamber size 22 litre
Lyla Sterilizer
supplied with reversible rack, 3 aluminium trays, drain tube, tray holder, 8 GB USB drive, door opening tool, factory test,
60 HzLyla 17
REF 19951250Chamber size 17 litre
Lyla 22 (220 V)
REF 19952250Chamber size 22 litre
Lyla 22 (110 V)
REF 19952350Chamber size 22 litre
Download Area
FAQ
- Which is the maximum noise level of Lyla?
- Stains, spots, or colour change on instruments.
- When starting a cycle, the chamber door locks but re-opens immediately. The “Open the door” message appears.
- The sterilizer enters into “Standby mode” immediately after being switched on.
- The sterilization (PROCESS) phase of a sterilization cycle was longer than expected.
- Warning about programmed maintenance.
- The cycle report printer does not work.
- The sterilizer is connected to an automated water supply system, there is no clean water in the tank and the automatic water filling does not fill the water.
- Warning about consumable replacement.
- Should I pouch/wrap my instruments and how long can I store them after sterilization?
- How do I clean the water tanks and what products should I use?
- How do I change consumable components on my sterilizer?
- Who should I contact to validate and maintain my sterilizer?
- Does my sterilizer need any periodic qualification/validation?
- How long should I archive the documentation of regular tests?
- Do I need to document the results of all periodic tests?
- What is validation?
- The sterilizer remains switched OFF.
- Water is leaking at the front of the sterilizer.
- At the end of the cycle, there is residual water on load.
- No cycles are stored in the cycle history menu.
- Do I need to carry out periodic testing on my W&H sterilizer?
- Warning about saving to the USB (HTML and SCL files)
- Which water quality should I use for my sterilizer?
Instructions for use
Find all languages of the Update Hygiene and Maintenance (EN ISO 17664:2017) in the Download Centre.
Videos & Tutorials
-
Lyla sterilization programs
Sterilization cycle S Wrapped 134 S Prion 134 S Gentle 121 S Unwrapped 134 Temperature: 134 °C 134 °C 121 °C 134 °C Holding time (minutes): 4 18' 30'' 20' 30'' 4 Total cycle duration
(minutes) including
complete drying:Automatic from Typical
loadAutomatic from Typical
loadAutomatic from Automatic from Typical
loadLyla 17 38' to 45'42' 52' to 59'56' 61' to 64' 20' to 26' 23' Lyla 22 (220 V) 39' to 46'43' 53' to 60'57' 62' to 66' 21' to 27' 24' Lyla 22 (110 V) 49' to 56'51' 63' to 71'65' 69' to 73
28' to 35' 30' Lyla 17/22 loads:maximum solid 4 kg/typical 2 kg/maximum porous 0.5 kgmaximum 4 kg unwrappedLyla 17/22 container load: maximum 4 kg not applicable Test cycles: Bowie and Dick/Vacuum test Item supplied with reversible rack, 3 aluminium trays, drain tube, tray holder, 8 GB USB drive, door opening tool, factory test
Lyla Sterilizer
Lyla 17 Lyla 22 Type: RIN-210 RIN-210 Chamber size: 17 l 22 l Power supply: 200–240 V AC; 50 Hz or 60 Hz; 10 A single phase
200–240 V AC; 50 Hz or 60 Hz; 10 A single phase
100–125 V AC; 50 Hz/60 Hz; 12 A single phase
Power consumption: 2.0–2.4 kW 220 V: 2.0–2.4 kW
110 V: 1.2–1.5 kWOverall dimension
(width × height × depth):465 × 452 × 646 mm Weight: 40 kg 42 kg Main / used water tanks: 4.8/4.8 l Working range: 7 to 10 cycles Usable space in chamber:
(width × height × depth)¹:195 × 195 × 312 mm 195 × 195 × 400 mm Connection types: 1 USB port in the front (additional 1 in the rear as option), automatic water filling kit (optional) (1) Usable space is referred to standard rack configuration.
Lyla sterilizers were designed, certified and validated with the most stringent directives and standards: N°2017/745 Medical Device Regulation 2014/68/EU Pressure Equipment Directive EN 13060 Small steam sterilizer IEC 61326-1 Electromagnetic compatibility IEC 61010-1 Safety requirements sterilizer
IEC 61010-2-040 Specific requirements for steam sterilizer
IEC 61770 Electric appliances connected to the water mains
The sterilizer can be validated according to EN ISO 17665-1










 Discover Product
Discover Product